Clinical Trial Interviews for Regulatory-Compliant Patient Experience Research
Clinigma® delivers regulatory-compliant clinical research services focused exclusively on in-trial patient interviews. We capture high-quality qualitative data that supports patient-focused drug development, strengthens regulatory submissions, and meets ICH-GCP standards for global trials.
Our commitment to listen to patients
CLINIGMA® was built on the belief that drug development and regulatory decision-making must consider what patients actually experience and value when taking new treatments. That’s why Jens Harald Kongsø founded CLINIGMA®.
Watch the video to hear more about our story and commitment to patient-focused drug development.

Interview
countries globally
Interview
languages available
Qualitative
researchers worldwide
Focused on
capturing patient feedback

Prix Galien Bridges award finalist—Best Medical Technology/AI
The CLINIGMA® Portal was selected as a finalist for the prestigious Prix Galien Bridges Award, the pharmaceutical industry's highest honor for innovation.
Trusted by leading pharmaceutical companies and recognized by partners and competitors alike as the best-in-class solution for capturing critical patient insights during clinical trials, the CLINIGMA® Portal ensures ICH-GCP compliance and transforms authentic patient experiences into regulatory-grade evidence.
Patient evidence for regulatory submissions
In-trial interviews
Transform your clinical trials with patient-centered insights. Our ICH-GCP compliant interview solutions capture meaningful patient experience data throughout the trial journey. From entry interviews to interim assessments and exit evaluations, we provide timely insights that strengthen strategic decision-making, regulatory submissions, and patient outcomes.
Real-world evidence
Generate compelling real-world evidence through rigorous qualitative research. We have expertise in standalone patient and expert interviews, and focus groups, outside of trials and in post-marketing contexts. We provide actionable insights for clear and informed decision-making. Tailoring solutions to your objectives, we use either IRB/EC-approved or traditional market research approaches.

Why patient voices matter
At CLINIGMA®, our expert team facilitates in-depth conversations with patients across all stages of drug development. We ensure regulatory agencies receive the rich, contextual evidence they need to make informed decisions. Discover how our approach transforms patient insights into compelling regulatory evidence.
Listen to our chief scientific officer, Anna Ryden (PhD), describe the value of qualitative interviews to provide an in-depth understanding of the patient experience in drug development.
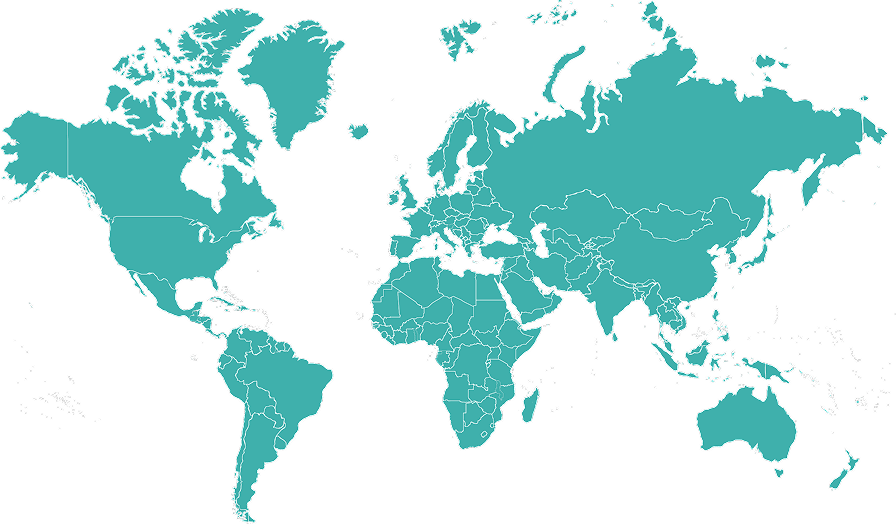
Global expertise, local knowledge
We operate across multiple regions, maintaining a global presence while adapting to specific local regulatory requirements. CLINIGMA® can seamlessly deliver consistently high quality ICH-GCP compliant interviews anywhere in the world.
Qualitative
researchers worldwide
Interview
languages available
The CLINIGMA® Portal:
for safe and secure patient interviews

Effortless scheduling
Intuitive portal designed for seamless interview coordination across global teams, ensuring optimal patient and researcher experiences.
Compliance
ICH-GCP compliant system with built-in data privacy protection in accordance with local regulations in EU, US, Japan, China, and other territories.
Secure data management
End-to-end encryption with long-term compliant storage solutions, maintaining data integrity for up to 25 years.
24/7 expert support
Dedicated team always on standby for queries and assistance, ensuring smooth project execution from start to finish.
CLINIGMA® team
ISO9001:2015 certified
CLINIGMA® consistently delivers excellence in interview services and solutions. Our systematic processes and continuous quality improvement are externally validated by ISO9001:2015. We are uniquely certified in the design, conduct, analysis, and reporting of patient interviews in relation to clinical trials. Our certification assures clients of our enduring commitment to quality, regulatory compliance, and innovation in the conduct and reporting of in-trial interviews.


Listen to your trial patients
Find out how our ICH-GCP compliant interview solutions can strengthen your drug development.












2.png)




