

REGULATORY BODIES WANT
PATIENT-EXPERIENCE DATA
Increasingly, regulatory agencies – led by the US Food and Drug Administration (FDA) and European Medicine Agency (EMA) – are acknowledging the value that patient feedback can contribute to medical product development. Following the FDA’s Patient-Focused Drug Development (PFDD) initiatives, the FDA have developed PFDD guidances to capture patient feedback in clinical trials and will already in 2021 start asking for patient-experience data to be included in new drug applications. In addition, the EMA will start developing similar PFDD guidances on patient preferences following the EMA regulatory science strategy 2025.
As a result of the expectations and requirements, it is expected that other regulatory agencies and HTAs will also begin requesting more detailed trial patient-experience data. For example, when assessing whether or not a new drug addresses the needs of a specific patient group. It is imperative that pharmaceutical companies are prepared to meet the increased expectations in clinical trial patient-experience data.
Clinigma® has the experience and know-how in interviewing patients in clinical trials in accordance with regulatory requirements.
CLINIGMA®
WE SUPPORT CLIENTS IN PATIENT-FOCUSED DRUG DEVELOPMENT
Clinigma® provides expert solutions in capturing patient feedback in clinical trials in accordance with the FDA PFDD guidances. Our global team of qualitative researchers have experience in gathering the critical insights that patients and caregivers bring to the medical product development process.
From an early stage in any clinical trial, patients and their caregivers are able to provide invaluable insights into how investigational drugs are experienced. This direct patient-experience feedback is set to change how medical products are developed, used and valued, and, in doing so, removing the uncertainties of drug development and approvals.
100%
FOCUS ON PATIENT INTERVIEWS IN CLINICAL TRIALS
Clinigma® is and, has been for several years, the only research company in the world exclusively specialized in interviewing clinical trial patients for the purpose of capturing and analyzing patient-experience data.
As the leader in the field of patient-interviews, we are active in discussions with regulatory agencies in Europe and the US on how best to incorporate patient feedback within the regulatory frameworks.
Clinigma® also has expertise in reporting back to patients on the results of their clinical trials as part of a patient-focused approach.
100%
FOCUS ON PATIENT INTERVIEWS IN CLINICAL TRIALS
Clinigma® is and, has been for several years, the only research company in the world exclusively specialized in interviewing clinical trial patients for the purpose of capturing and analyzing patient-experience data.
As the leader in the field of patient-interviews, we are active in discussions with regulatory agencies in Europe and the US on how best to incorporate patient feedback within the regulatory frameworks.
Clinigma® also has expertise in reporting back to patients on the results of their clinical trials as part of a patient-focused approach.
200+
QUALITATIVE RESEARCHERS WORLDWIDE
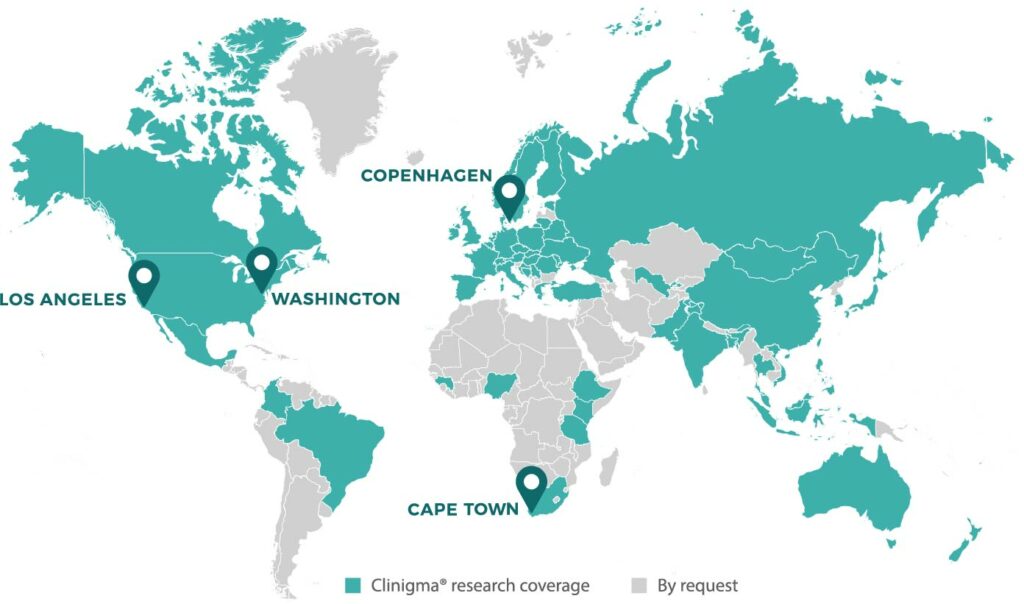
Clinigma® has the largest network of qualitative researchers on the market, making it easy for us to adjust for changes in country allocations and to offer in-person interviews in almost any country. Our global qualitative researchers are highly educated within anthropology, psychology, sociology or similar education and have undergone GCP training prior to interviewing patients in clinical trials.
Clinigma® can moderate interviews in more than 70 languages.
Clinigma® is currently conducting qualitative patient interviews in global clinical trials across the world – in the US, Europe and Asia.
CLINIGMA® PORTAL
FACILITATING THE PATIENT INTERVIEW PROCESS
Clinigma® has developed a unique IT portal removing the hassle of conducting patient interviews in global clinical trials.
Our portal ensures easy and secure collection of patient-experience data while facilitating communication between the Clinigma® research team, our clients, clinical sites and clinical trial participants.
“The Clinigma® Portal matches the current threat environment of web application and can withstand state-of-the-art security threats”
– NCC Group
CLINIGMA® PORTAL
FACILITATING THE PATIENT INTERVIEW PROCESS
Clinigma® has developed a unique IT portal removing the hassle of conducting patient interviews in global clinical trials.
Our portal ensures easy and secure collection of patient-experience data while facilitating communication between the Clinigma® research team, our clients, clinical sites and clinical trial participants.
“The Clinigma® Portal matches the current threat environment of web application and can withstand state-of-the-art security threats”
– NCC Group
ISO 9001
CERTIFIED
Clinigma® provides high-quality services to clients in the pharmaceutical industry. To demonstrate this, we had a quality assurance and risk management certification body, DNV-GL, to perform an ISO certification assessment resulting in Clinigma® getting ISO 9001:2015 certified in February 2021.
Clinigma® is ISO 9001:2015 certified in:
“Designing, conducting, analysing and reporting patient interviews in relation to clinical trials”
