

OUR INSIGHTS

Are you developing drugs in accordance with regulatory guidances on Patient-Focused Drug Development?
The FDA and the EMA are strengthening Patient-Focused Drug Development. The US Food and Drug Administration (FDA) and the European Medicines Agency (EMA) both acknowledge that…
Read More
Why PROMs do not guarantee a full picture of patients’ experiences with new drugs
The limitations of Patient Reported Outcome Measures (PROMs). Traditional PROMs (consisting of closed-end questions) have for many years been applied to reveal the patients’ experiences of effects…
Read More
Can you afford to launch a new drug without hearing what patients think about it?
Get the full picture of a treatment. To ensure that you get a clearer picture of a treatment, we recommend interviewing the users who are exposed to a drug candidate as early as possible in the trial…
Read More
Introducing the Clinigma® Portal
An IT portal facilitating secure and easy collection of trial patient feedback. Patient insights and experiences are critical for pharmaceutical companies to successfully bring new drugs to market…
Read More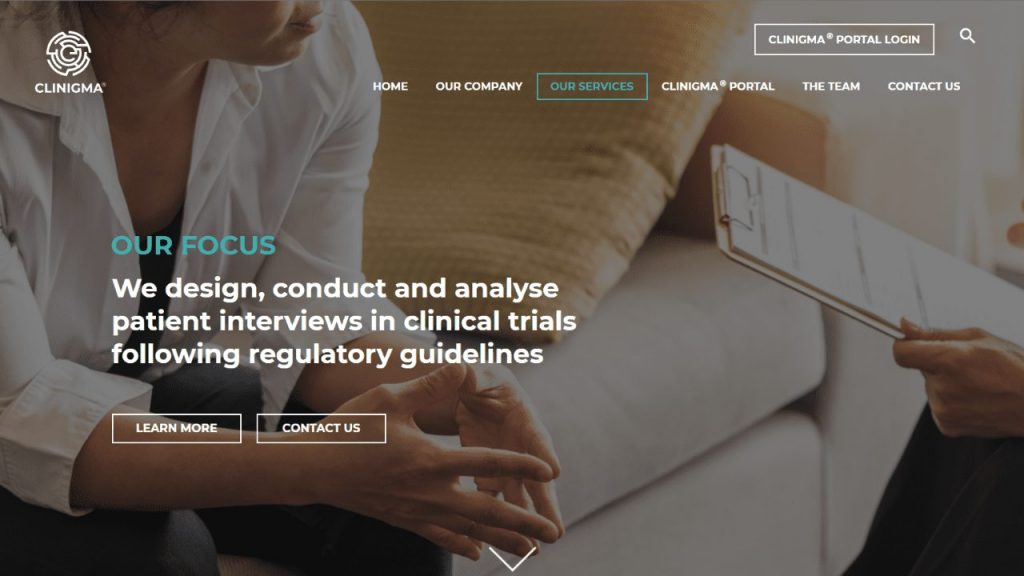
Our new website is live!
Visit the new Clinigma® website today. We are very excited to announce that our new and refreshed website www.clinigma.com is now live. The updated site includes all the information you need to learn more…
Read More
The importance of listening to the patients in clinical trials
Increasing use of qualitative research methods in clinical trials. At Clinigma®, we are not the only ones recognizing the value of listening to the patients in clinical trials. Increasingly, regulatory agencies…
Read More